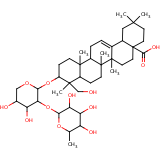
| Compound Information | SONAR Target prediction | | Name: | SAPINDOSIDE A | | Unique Identifier: | SPE01504017 | | MolClass: | Checkout models in ver1.5 and ver1.0 | | Molecular Formula: | | | Molecular Weight: | 684.432 g/mol | | X log p: | 0.138000000000001 (online calculus) | | Lipinksi Failures | 1 | | TPSA | 53.99 | | Hydrogen Bond Donor Count: | 0 | | Hydrogen Bond Acceptors Count: | 12 | | Rotatable Bond Count: | 6 | | Canonical Smiles: | CC1OC(OC2C(O)C(O)COC2OC2CCC3(C)C(CCC4(C)C3CC=C3C5CC(C)(C)CCC5(CCC34C)C
(O)=O)C2(C)CO)C(O)C(O)C1O | | Class: | triterpene glycoside | | Source: | Anemone coronaria, Sapindus, Hedera, Astrantia spp; alpha-hederin | | Reference: | Annalen 726:125 (1969); Chem Nat Compd (Eng Trans) 6:213, 316, 380, 440 (1970);
8:468 (1972) |
| Species: |
4932 |
| Condition: |
CIN8 |
| Replicates: |
2 |
| Raw OD Value: r im |
0.3540±0.0997021 |
| Normalized OD Score: sc h |
0.2535±0.122969 |
| Z-Score: |
-30.1322±6.2757 |
| p-Value: |
0 |
| Z-Factor: |
0.261712 |
| Fitness Defect: |
INF |
| Bioactivity Statement: |
Active |
| Experimental Conditions | | | Library: | Spectrum | | Plate Number and Position: | 6|C4 | | Drug Concentration: | 50.00 nM | | OD Absorbance: | 600 nm | | Robot Temperature: | 25.70 Celcius | | Date: | 2006-02-24 YYYY-MM-DD | | Plate CH Control (+): | 0.039099999999999996±0.00095 | | Plate DMSO Control (-): | 0.7706000000000001±0.01012 | | Plate Z-Factor: | 0.9563 |
|  png png
ps
pdf |
| 11700570 |
(4aS,6aR,6aS,6bR,8aR,9S,10S,12aS,14bR)-10-[(2S,3R,4S,5R)-4,5-dihydroxy-3-[(2R,3R,4S,5S,6R)-3,4,5-trihydr
oxy-6-(hydroxymethyl)oxan-2-yl]oxy-oxan-2-yl]oxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,
6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid |
| internal high similarity DBLink | Rows returned: 0 | |
| active | Cluster 15543 | Additional Members: 5 | Rows returned: 2 | |
|
