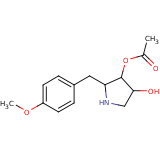
| Compound Information | SONAR Target prediction | | Name: | ANISOMYCIN | | Unique Identifier: | SPE01503906 | | MolClass: | Checkout models in ver1.5 and ver1.0 | | Molecular Formula: | | | Molecular Weight: | 246.154 g/mol | | X log p: | 8.199 (online calculus) | | Lipinksi Failures | 1 | | TPSA | 35.53 | | Hydrogen Bond Donor Count: | 0 | | Hydrogen Bond Acceptors Count: | 5 | | Rotatable Bond Count: | 5 | | Canonical Smiles: | COc1ccc(CC2NCC(O)C2OC(C)=O)cc1 | | Class: | alkaloid | | Source: | Streptomyces griseolus | | Reference: | JACS 76:4053 (1954); Chem Pharm Bull 17:1405 (1969); Prog Neurobiology 16:155
(1981); Mol Cell Biochem 14:7352 (1994) | | Therapeutics: | antiprotozoal, antifungal, protein synthesis inhibitor |
| Species: |
4932 |
| Condition: |
UBP8 |
| Replicates: |
2 |
| Raw OD Value: r im |
0.4475±0.0351432 |
| Normalized OD Score: sc h |
0.7233±0.0532811 |
| Z-Score: |
-11.4022±2.1902 |
| p-Value: |
3.31036e-23 |
| Z-Factor: |
-0.348665 |
| Fitness Defect: |
51.7624 |
| Bioactivity Statement: |
Active |
| Experimental Conditions | | | Library: | Spectrum | | Plate Number and Position: | 17|F9 | | Drug Concentration: | 50.00 nM | | OD Absorbance: | 600 nm | | Robot Temperature: | 22.80 Celcius | | Date: | 2007-10-17 YYYY-MM-DD | | Plate CH Control (+): | 0.0413±0.00127 | | Plate DMSO Control (-): | 0.6126±0.04145 | | Plate Z-Factor: | 0.7547 |
|  png png
ps
pdf |
| 2199 |
[4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate |
| 31549 |
[(2S,3R,4R)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate |
| 253602 |
[(2R,3S,4S)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate |
| 462385 |
[(2S,3R,4R)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate hydrochloride |
| 1268331 |
[(2R,3R,4R)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate |
| 5458313 |
[(2S,3R,4R)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrolidin-3-yl] acetate chloride |
| internal high similarity DBLink | Rows returned: 0 | |
| active | Cluster 6951 | Additional Members: 3 | Rows returned: 0 | |
|
