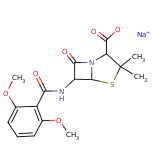
| Compound Information | SONAR Target prediction | | Name: | METHICILLIN SODIUM | | Unique Identifier: | SPE01500395 | | MolClass: | Checkout models in ver1.5 and ver1.0 | | Molecular Formula: | | | Molecular Weight: | 383.248 g/mol | | X log p: | 5.686 (online calculus) | | Lipinksi Failures | 1 | | TPSA | 121.27 | | Hydrogen Bond Donor Count: | 0 | | Hydrogen Bond Acceptors Count: | 8 | | Rotatable Bond Count: | 6 | | Canonical Smiles: | [Na+].[O-]C(=O)C1N2C(SC1(C)C)C(NC(=O)c1c(OC)cccc1OC)C2=O | | Source: | semisynthetic | | Therapeutics: | antibacterial |
| Species: |
4932 |
| Condition: |
UBP8 |
| Replicates: |
2 |
| Raw OD Value: r im |
0.6860±0.0224153 |
| Normalized OD Score: sc h |
1.0063±0.000230357 |
| Z-Score: |
0.2598±0.00936683 |
| p-Value: |
0.794996 |
| Z-Factor: |
-6.61478 |
| Fitness Defect: |
0.2294 |
| Bioactivity Statement: |
Nonactive |
| Experimental Conditions | | | Library: | Spectrum | | Plate Number and Position: | 20|H11 | | Drug Concentration: | 50.00 nM | | OD Absorbance: | 600 nm | | Robot Temperature: | 23.10 Celcius | | Date: | 2007-10-17 YYYY-MM-DD | | Plate CH Control (+): | 0.03995±0.00097 | | Plate DMSO Control (-): | 0.6712750000000001±0.01409 | | Plate Z-Factor: | 0.9241 |
|  png png
ps
pdf |
| 1548978 |
(2R,5R,6S)-6-[(2,6-dimethoxybenzoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbox
ylate |
| 1548979 |
(2R,5R,6S)-6-[(2,6-dimethoxybenzoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbox
ylic acid |
| 1801713 |
(2S,5R,6S)-6-[(2,6-dimethoxybenzoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbox
ylate |
| 1801714 |
(2S,5R,6S)-6-[(2,6-dimethoxybenzoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbox
ylic acid |
| 6560175 |
(2S,5S,6R)-6-[(2,6-dimethoxybenzoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbox
ylate |
| 6560176 |
(2S,5S,6R)-6-[(2,6-dimethoxybenzoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbox
ylic acid |
| internal high similarity DBLink | Rows returned: 0 | |
| active | Cluster 3276 | Additional Members: 16 | Rows returned: 1 | |
|
