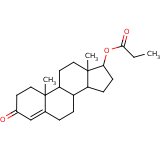
| Compound Information | SONAR Target prediction | | Name: | TESTOSTERONE PROPIONATE | | Unique Identifier: | SPE00300034 | | MolClass: | Checkout models in ver1.5 and ver1.0 | | Molecular Formula: | | | Molecular Weight: | 312.234 g/mol | | X log p: | 1.957 (online calculus) | | Lipinksi Failures | 0 | | TPSA | 43.37 | | Hydrogen Bond Donor Count: | 0 | | Hydrogen Bond Acceptors Count: | 3 | | Rotatable Bond Count: | 3 | | Canonical Smiles: | CCC(=O)OC1CCC2C3CCC4=CC(=O)CCC4(C)C3CCC12C | | Source: | semisynthetic | | Therapeutics: | androgen, antineoplastic |
| Species: |
4932 |
| Condition: |
SPE00330001 |
| Replicates: |
2 |
| Raw OD Value: r im |
0.0406±0 |
| Normalized OD Score: sc h |
0.1331±0 |
| Z-Score: |
-9.8090±0 |
| p-Value: |
1.03037e-22 |
| Z-Factor: |
0.595426 |
| Fitness Defect: |
50.627 |
| Bioactivity Statement: |
Toxic |
| Experimental Conditions | | | Library: | SpectrumTMP | | Plate Number and Position: | 1|E10 | | Drug Concentration: | 50.00 nM | | OD Absorbance: | 600 nm | | Robot Temperature: | 0.00 Celcius | | Date: | 2006-08-17 YYYY-MM-DD | | Plate CH Control (+): | 0.0387±0.00132 | | Plate DMSO Control (-): | 0.3051±0.03481 | | Plate Z-Factor: | 0.5824 |
|  png png
ps
pdf |
| 4554800 |
(7,10,13-trimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl)
propanoate |
| 5315133 |
[(6bS,11R)-4,4,6a,6b,8a,11,12,14b-octamethyl-14-oxo-1,2,3,4a,5,6,7,8,9,10,11,12,12a,14a-tetradecahydropi
cen-3-yl] octacosanoate |
| 5701990 |
[(10R,13S,17S)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17
-yl] propanoate |
| 5748262 |
[(8R,9R,10S,13S,14R,17R)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phen
anthren-17-yl] propanoate |
| 6427041 |
(13-methyl-3-oxo-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)
dodecanoate |
| 6432323 |
[(17S)-10,13-dimethyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl]
heptanoate |
| internal high similarity DBLink | Rows returned: 5 | |
| active | Cluster 2094 | Additional Members: 20 | Rows returned: 6 | |
|
