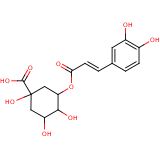
| Compound Information | SONAR Target prediction | | Name: | CHLOROGENIC ACID | | Unique Identifier: | SPE00210800 | | MolClass: | Checkout models in ver1.5 and ver1.0 | | Molecular Formula: | | | Molecular Weight: | 336.166 g/mol | | X log p: | 8.313 (online calculus) | | Lipinksi Failures | 1 | | TPSA | 43.37 | | Hydrogen Bond Donor Count: | 0 | | Hydrogen Bond Acceptors Count: | 9 | | Rotatable Bond Count: | 5 | | Canonical Smiles: | OC1CC(O)(CC(OC(=O)C=Cc2ccc(O)c(O)c2)C1O)C(O)=O | | Class: | aromatic | | Source: | occurence in many plants | | Reference: | Phytochemistry 15: 703 (1976); Biochem J 361: 57 (2002) | | Therapeutics: | antioxidant, free radical scavenger |
| Species: |
4932 |
| Condition: |
TOR1 |
| Replicates: |
2 |
| Raw OD Value: r im |
0.9575±0.00289914 |
| Normalized OD Score: sc h |
0.9473±0.00392224 |
| Z-Score: |
-0.2794±0.0591961 |
| p-Value: |
0.78016 |
| Z-Factor: |
-1.33862 |
| Fitness Defect: |
0.2483 |
| Bioactivity Statement: |
Nonactive |
| Experimental Conditions | | | Library: | Spectrum_ED | | Plate Number and Position: | 16|E7 | | Drug Concentration: | 50.00 nM | | OD Absorbance: | 595 nm | | Robot Temperature: | 30.00 Celcius | | Date: | 2012-09-18 YYYY-MM-DD | | Plate CH Control (+): | -0.00025±0.00199 | | Plate DMSO Control (-): | 1.00045±0.03683 | | Plate Z-Factor: | 0.8780 |
|  png png
ps
pdf |
| 9476 |
(1R,3R,4S,5R)-3-[3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]-1,4,5-trihydroxy-cyclohexane-1-carboxylic acid |
| 73081 |
(1R,3R,4R,5R)-3-[3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]-1,4,5-trihydroxy-cyclohexane-1-carboxylic acid |
| 91492 |
(1S,3R,4R,5R)-3-[3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]-1,4,5-trihydroxy-cyclohexane-1-carboxylic acid |
| 348159 |
3-[3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]-1,4,5-trihydroxy-cyclohexane-1-carboxylic acid |
| 443706 |
(3R,4S,5R)-3-[3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]-1,4,5-trihydroxy-cyclohexane-1-carboxylic acid |
| 1794427 |
(1R,3R,4S,5R)-3-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,4,5-trihydroxy-cyclohexane-1-carboxylic
acid |
| internal high similarity DBLink | Rows returned: 0 | |
| active | Cluster 6023 | Additional Members: 3 | Rows returned: 0 | |
|
