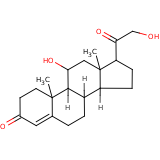
| Compound Information | SONAR Target prediction | | Name: | Corticosterone | | Unique Identifier: | LOPAC 00759 | | MolClass: | Checkout models in ver1.5 and ver1.0 | | Molecular Formula: | C21H30O4 | | Molecular Weight: | 319.246 g/mol | | X log p: | 0.527 (online calculus) | | Lipinksi Failures | 0 | | TPSA | 34.14 | | Hydrogen Bond Donor Count: | 0 | | Hydrogen Bond Acceptors Count: | 4 | | Rotatable Bond Count: | 2 | | Canonical Smiles: | CC12CC(O)C3C(CCC4=CC(=O)CCC34C)C1CCC2C(=O)CO | | Class: | Hormone | | Selectivity: | Glucocorticoid |
| Species: |
4932 |
| Condition: |
BY4741 |
| Replicates: |
8 |
| Raw OD Value: r im |
0.7966±0.115166 |
| Normalized OD Score: sc h |
1.0117±0.00694489 |
| Z-Score: |
0.3613±0.20624 |
| p-Value: |
0.717894 |
| Z-Factor: |
-5.14132 |
| Fitness Defect: |
0.3314 |
| Bioactivity Statement: |
Nonactive |
| Experimental Conditions | | | Library: | Lopac | | Plate Number and Position: | 3|F11 | | Drug Concentration: | 50.00 nM | | OD Absorbance: | 600 nm | | Robot Temperature: | 27.60 Celcius | | Date: | 2005-04-07 YYYY-MM-DD | | Plate CH Control (+): | 0.045962499999999996±0.00136 | | Plate DMSO Control (-): | 0.7777812499999999±0.02984 | | Plate Z-Factor: | 0.8704 |
|  png png
ps
pdf |
| 4122713 |
8-hydroxy-17-(2-hydroxyacetyl)-13-methyl-1,2,6,7,9,10,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanth
ren-3-one |
| 5316088 |
(4aR,5S,6aR,7S)-5-hydroxy-7-(2-hydroxyacetyl)-4a,6a-dimethyl-4,4b,5,6,7,8,9,10,10a,10b,11,12-dodecahydro
-3H-chrysen-2-one |
| 6540812 |
(8S,9R,10R,11S,13S,14S,17R)-11-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17
-dodecahydrocyclopenta[a]phenanthren-3-one |
| 6603769 |
(8S,9S,10S,11R,13S,14R,17R)-11-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17
-dodecahydrocyclopenta[a]phenanthren-3-one |
| 6918887 |
(8R,9S,10S,11R,13R,14R,17S)-11-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17
-dodecahydrocyclopenta[a]phenanthren-3-one |
| internal high similarity DBLink | Rows returned: 6 | |
|
