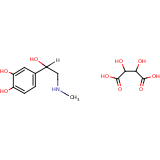
| Compound Information | SONAR Target prediction | | Name: | (-)-Epinephrine bitartrate | | Unique Identifier: | LOPAC 00114 | | MolClass: | Checkout models in ver1.5 and ver1.0 | | Molecular Formula: | C13H19NO9 | | Molecular Weight: | 315.148 g/mol | | X log p: | 6.036 (online calculus) | | Lipinksi Failures | 1 | | TPSA | 0 | | Hydrogen Bond Donor Count: | 0 | | Hydrogen Bond Acceptors Count: | 4 | | Rotatable Bond Count: | 3 | | Canonical Smiles: | CNCC(O)c1ccc(O)c(O)c1.OC(C(O)C(O)=O)C(O)=O | | Class: | Adrenoceptor | | Action: | Agonist |
| Species: |
4932 |
| Condition: |
PAC10 |
| Replicates: |
2 |
| Raw OD Value: r im |
0.7191±0.00247487 |
| Normalized OD Score: sc h |
1.0241±0.00199394 |
| Z-Score: |
1.0935±0.0767948 |
| p-Value: |
0.274896 |
| Z-Factor: |
-3.73816 |
| Fitness Defect: |
1.2914 |
| Bioactivity Statement: |
Nonactive |
| Experimental Conditions | | | Library: | Lopac | | Plate Number and Position: | 7|C3 | | Drug Concentration: | 50.00 nM | | OD Absorbance: | 600 nm | | Robot Temperature: | 27.60 Celcius | | Date: | 2005-04-15 YYYY-MM-DD | | Plate CH Control (+): | 0.044599999999999994±0.00168 | | Plate DMSO Control (-): | 0.66195±0.02552 | | Plate Z-Factor: | 0.8567 |
|  png png
ps
pdf |
| DBLink | Rows returned: 5 | |
| 5815 |
(2R,3R)-2,3-dihydroxybutanedioic acid; 4-[(1R)-1-hydroxy-2-methylamino-ethyl]benzene-1,2-diol |
| 89249 |
(2R,3R)-2,3-dihydroxybutanedioic acid; 4-(1-hydroxy-2-methylamino-ethyl)benzene-1,2-diol |
| 5702049 |
2,3-dihydroxybutanedioic acid; 4-[(1S)-1-hydroxy-2-methylamino-ethyl]benzene-1,2-diol |
| 6604103 |
(2R,3S)-2,3-dihydroxybutanedioic acid; 4-[(1R)-1-hydroxy-2-methylamino-ethyl]benzene-1,2-diol |
| 6852374 |
2,3-dihydroxybutanedioic acid; 4-[(1R)-1-hydroxy-2-methylamino-ethyl]benzene-1,2-diol |
| internal high similarity DBLink | Rows returned: 5 | |
|
